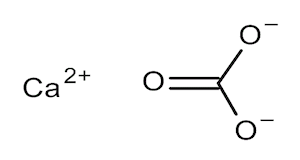
Calcium Carbonate
Also known as Calcite, Chalk
| H.S.Code | 28365000 | CAS No. | 471-34-1 |
| Molecular Formula | CaCO3 | Molecular Weight | 100.09 |
| Physical State | Solid | Refractive index | 1.59 |
| Odour | Odorless | Insoluble in water | |
| Density | 2.711 g/cm³ | Flash point | |
| pH value | 8 | Melting Point | 825 °C |
About Calcium Carbonate
Calcium Carbonate is a common substance found in rocks as the minerals calcite and aragonite which is a type of sedimentary rock consisting mainly of calcite. Calcium carbonate is the active ingredient in agricultural lime and is created when calcium ions in hard water react with carbonate ions to create limescale. The vast majority of calcium carbonate used in industry is extracted by mining or quarrying. Pure calcium carbonate can be produced from a pure quarried source.
Calcium Carbonate has medical use as a calcium supplement or as an antacid. The main use of calcium carbonate is in the construction industry, either as a building material, or limestone aggregate for road building, or as an ingredient of cement. Calcium carbonate is also used in the purification of iron from iron ore in a blast furnace. Calcium carbonate in the form of chalk has traditionally been a major component of blackboard chalk.
Calcium Carbonate Specifications
| Packaging | 500 gm |
| Form | Powder |
| Colour | White |
| Grade | LR / AR |
| Hazard Class | |
| Storage | Store in a cool, dry area |
| Shelf Life | 12 Months |

500 ml