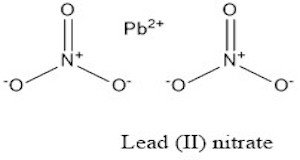
Lead Nitrate
Also known as Lead (II) nitrate, Lead dinitrate
| H.S.Code | 28342920 | CAS No. | 10099-74-8 |
| Molecular Formula | N2O6Pb | Molecular Weight | 331.21 |
| Physical State | Solid | Refractive index | 1.782 |
| Odour | Odorless | Solubility in water | Soluble in water |
| Density | 4.53 g/cm³ | Flash point | |
| pH value | Melting Point | 470 °C |
About Lead Nitrate
Commonly occurs as a colourless crystal or white powder and, unlike most other lead(II) salts, is soluble in water. Lead nitrate is produced by reaction of lead(II) oxide with concentrated nitric acid. It may also be obtained evaporation of the solution obtained by reacting metallic lead with dilute nitric acid. Lead nitrate is toxic and must be handled with care to prevent inhalation, ingestion and skin contact.
Lead nitrate has been used as a heat stabiliser in nylon and polyesters, as a coating for photothermographic paper, and in rodenticides. Due to its hazardous nature, the limited applications of lead(II) nitrate are under constant scrutiny.
Lead Nitrate Specifications
| Packaging | 500 gm |
| Form | Crystal / Powder |
| Colour | White |
| Grade | LR/AR |
| Hazard Class | 5.1 |
| Storage | Store in a cool, dry, well-ventilated place. |
| Shelf Life | 60 Months |

500 gm