Laboratory Chemical Search
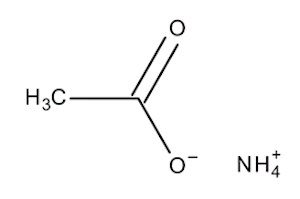
Ammonium Acetate (For Molecular Biology)
Also known as Ammonium ethanoate, Ammonium salt
| H.S.Code | 29152990 | CAS No. | 631-61-8 |
| Molecular Formula | C2H4O2.H3N | Molecular Weight | 77.08 |
| Physical State | Solid | Refractive index | |
| Odour | Slightly Acetic | Solubility in water | Soluble |
| Density | 1.17 g/cm3 | Flash point | 136 °C |
| pH value | 6.5 - 7.5 | Melting Point | 112 °C |
About Ammonium Acetate
Ammonium Acetate is a white, hygroscopic solid and can be derived from the reaction of ammonia and acetic acid. Ammonium acetate is produced by the neutralization of acetic acid with ammonium carbonate or by saturating glacial acetic acid with ammonia.
Ammonium Acetate is used to replace cell buffers that contain non-volatile salts in preparing samples for mass spectrometry. It is also popular as a buffer for mobile phases for HPLC with ELSD detection.
Ammonium Acetate Specifications
| Packaging | 500 gm |
| Form | Crystals |
| Colour | White |
| Grade | AR/ACS/HPLC |
| Hazard Class | |
| Storage | Keep container tightly closed in a dry and well-ventilated place. |
| Shelf Life | 24 Months |

500 ml